Guidelines
Lorem ipsum dolor sit amet, consectetur adipiscing elit ut aliquam, purus sit amet luctus venenatis, lectus magna fringilla urna, porttitor rhoncus dolor purus non

Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update,
JASE, March 2018
Summary of FDA Approved Applications for Contrast document

Guidelines for the Cardiac Sonographer in the Performance of Contrast Echocardiography: A Focused Update from the American Society of Echocardiography
JASE, August 2014

American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography
JASE, November 2008

In an effort to help improve quality and encourage the appropriate use of ultrasound contrast, ASE established the ContrastZone website to provide the cardiovascular ultrasound community with a central place on the web to find both basic and advanced information about Ultrasound Enhancing Agents (UEA), also known as ultrasound contrast. This site includes links to recent ASE guidelines and standards, how-to videos, reimbursement information, and success stories from busy labs on how to incorporate UEAs into everyday practice to improve overall quality. Explore the menu to learn more!
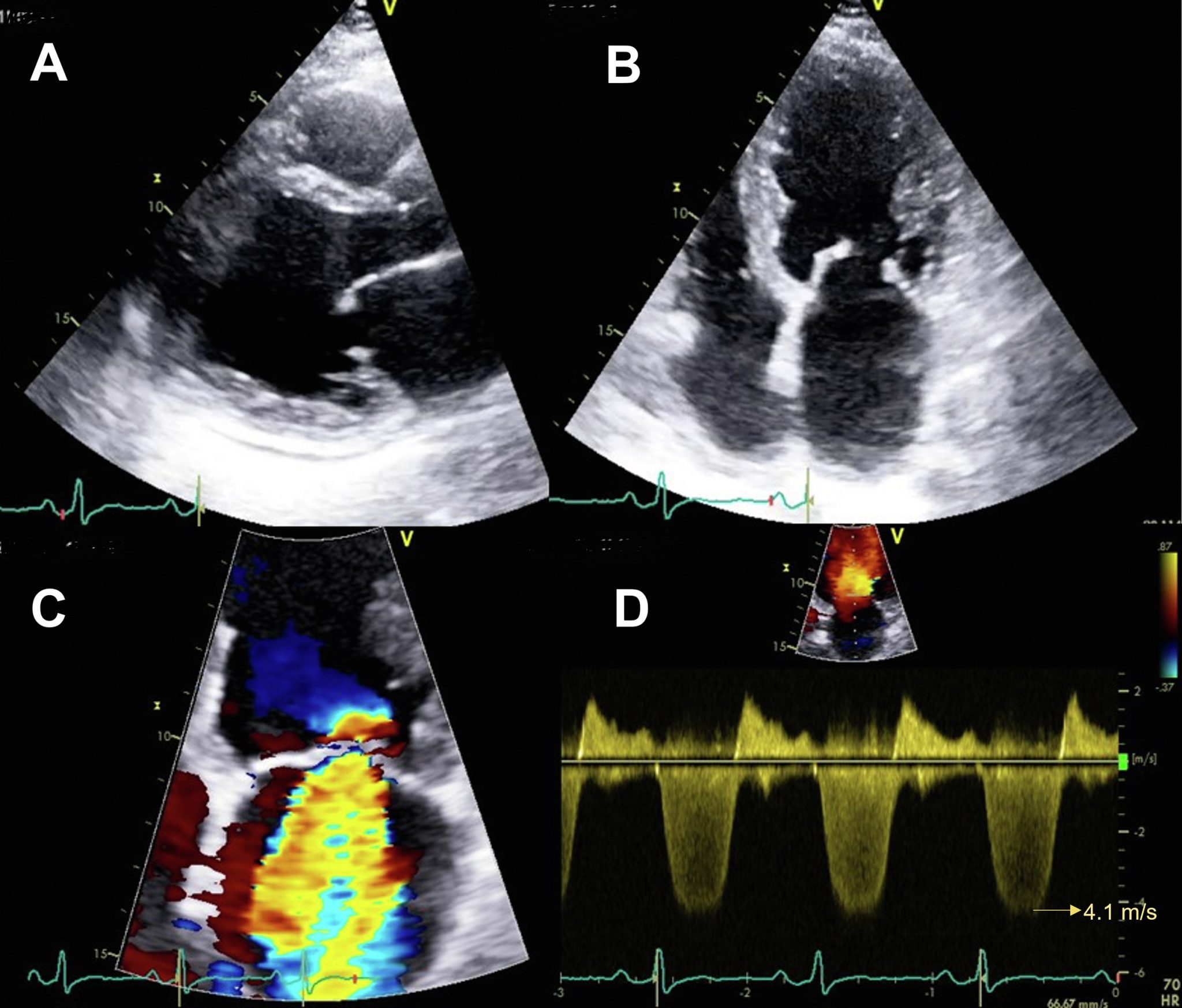
An example of a stress-induced perfusion defect in the left anterior descending coronary artery (LAD) territory (arrows). Note that end-systolic replenishment within the LAD territory in the apical four-chamber window is normal under resting conditions but delayed in the LAD territory (arrows) during dobutamine stress imaging. Figure 13 from “Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update”, JASE, March 2018
Resources
Ultrasound Enhancing Agents: Recommended Laboratory Practices from ASE
Helpful Information from Industry
Coding for Contrast
Videos and Webinars
How to Administer Contrast
Michelle Bierig, RDCS, FASE and Jeanetta Murphey, RN, BSN from St. Anthony’s Medical Center demonstrate the three various methods for bolus injection.
Ultrasound Enhancing Agent Safety
Michelle Bierig, RDCS, FASE and Jeanetta Murphey, RN, BSN from St. Anthony’s Medical Center demonstrate the three various methods for bolus injection.
How to Perform a Myocardial Perfusion Study
Kevin Wei, MD, FASE from Oregon Health and Sciences Center in Portland,OR
discusses how to perform a myocardial perfusion study.
How to Perform a Myocardial Perfusion Study: Part 1 Introduction
Dr. Wei demonstrates how to prepare the contrast media for use in an cardiac ultrasound study.
How to Perform a Myocardial Perfusion Study: Part 2 Components
Dr. Wei shows how to have materials prepared prior to using contrast media for use in an cardiac ultrasound study.
How to Perform a Myocardial Perfusion Study: Part 3 Diluting Contrast Media
Dr. Wei walks us through how to prepare contrast media for injection prior to a cardiac ultrasound study.
How to Perform a Myocardial Perfusion Study: Part 4 Pump
Dr. Wei demonstrates how to pump contrast media during a cardiac ultrasound study.
How to Perform a Myocardial Perfusion Study: Part 5 Machine Settings
Dr. Wei instructs how to set up an ultrasound machine to maximize the effectiveness of contrast media during a cardiac ultrasound study.
How to Perform a Myocardial Perfusion Study: Part 6 Manual Settings
Dr. Wei instructs how to set up an ultrasound machine without automatic presets to maximize the effectiveness of contrast media during a cardiac ultrasound study.
Basics of Bubbles: What Every Clinician Should Know:
This educational program provides key information for both early users and established users of ultrasound enhancing agents and is intended to promote the adoption and appropriate use of contrast in echocardiography more broadly. The content of the lectures has been carefully selected by top experts in the field to provide valuable information regarding the contrast agents available, the methods used to detect them, policies and procedures necessary for integration of contrast echocardiography, appropriate indications for contrast using case-based approaches, and advanced uses such as perfusion imaging. The use of physician-sonographer teams in the live program is intended to stress the need for team-based implantation of contrast policies and procedures.
FAQs
The Basics
Contrast-enhanced ultrasound (CEUS) is the application of an ultrasound contrast medium to traditional medical sonography. Ultrasound contrast agents rely on the different ways in which sound waves are reflected from interfaces between substances. This may be the surface of a small air bubble or a more complex structure. Commercially available contrast media are gas-filled microbubbles that are administered intravenously to the systemic circulation. Microbubbles have a high degree of echogenicity, which is the ability of an object to reflect ultrasound waves. The difference in echogenicity between the gas in the microbubbles and the soft tissue surrounding the body is immense. Thus, ultrasonic imaging using microbubble contrast agents enhances the ultrasound backscatter, or reflection of the ultrasound waves, to produce a unique echocardiogram with increased contrast, owing to the high echogenicity difference. Contrast-enhanced ultrasound can be used to image blood perfusion in organs (Figure 1), measure blood flow rate in the heart and other organs, and has other applications as well.
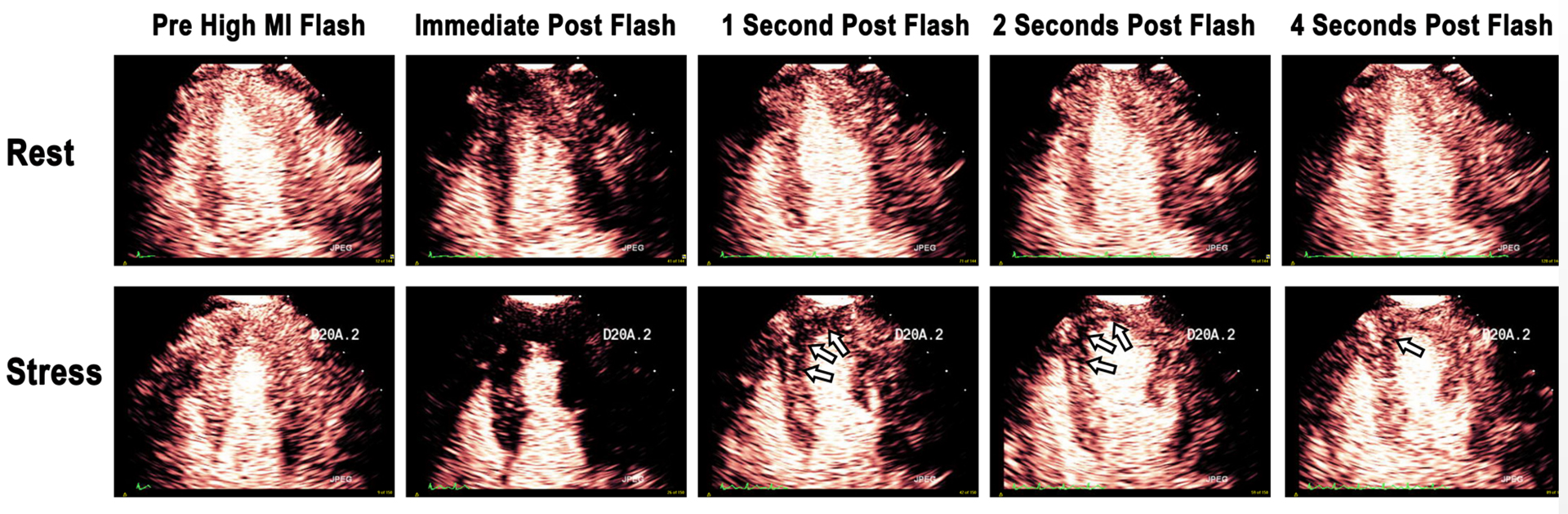
Figure 1. Contrast enhanced echocardiographic images obtained immediately after a high mechanical index impulse (i), one second after (ii), two seconds after (iii), and four seconds after the high mechanical index impulse (iv).
What is a microbubble?
There are a variety of microbubble contrast agents. Microbubbles differ in their shell makeup, gas core makeup, and whether or not they are targeted.
Microbubble shell: The composition of its shell material determines how easily a microbubble is taken up by the immune system. A more hydrophilic material tends to be taken up more easily, which reduces the microbubble’s residence time in the circulation. This reduces the time available for contrast imaging. The shell material also affects the mechanical elasticity of the microbubble. The more elastic the material, the more acoustic energy it can withstand before bursting. Currently, microbubble shells are composed of albumin, galactose, lipids, or polymers.
Microbubble gas core: The gas core is the most important part of the ultrasound contrast microbubble because it determines its echogenicity. When gas bubbles are caught in an ultrasonic frequency field, they compress, oscillate, and reflect a characteristic echo–this generates the strong and unique sonogram characteristic of contrast-enhanced ultrasound. Gas cores can be composed of air, heavy gases like perfluorocarbon, or nitrogen. Heavy gases are less water-soluble, so they are less likely to leak out from the microbubble and impair echogenicity. Therefore, microbubbles with heavy gas cores are likely to last longer in circulation.
Regardless of the shell or gas core composition, microbubble size is fairly uniform. It spans a range of 1-4 microns in diameter. That makes microbubbles smaller than red blood cells, which allows them to flow easily through the circulation as well as the microcirculation.
Who makes contrast media?
GE Healthcare makes Optison, a Food and Drug Administration (FDA)-approved microbubble. It has an albumin shell and octafluoropropane gas core.
Lantheus makes Definity, which is a FDA-approved microbubble. In this product, perflutren lipid microspheres composed of octafluoropropane encapsulated in an outer lipid shell.
Bracco makes Lumason (SonoVue in other countries) which is also FDA-approved. It is a sulfur hexafluoride containing lipid-encapsulated microbubble.
How it works
There are two forms of contrast-enhanced ultrasound or CEUS: untargeted (used in the clinic today) and targeted (under preclinical development). The two methods differ slightly from one another.
Untargeted CEUS
All FDA-approved microbubbles are untargeted. They are injected intravenously into the systemic circulation in a small bolus or as a dilute continuous infusion. The microbubbles will remain in the systemic circulation for a certain period of time. During that time, ultrasound waves are directed toward the area of interest. When microbubbles in the blood flow pass the imaging window, the microbubbles’ compressible gas cores oscillate in response to the high frequency sonic energy field. The microbubbles reflect a unique echo that stands in stark contrast to the surrounding tissue, owing to the discrepancy in orders of magnitude between microbubble and tissue echogenicity. The ultrasound system converts the strong echogenicity into a contrast-enhanced image of the area of interest. In this way, the bloodstream’s echo is enhanced, allowing the clinician to distinguish blood from surrounding tissues.
In order to optimize microbubble contrast and reduce noise from tissue, ultrasound manufacturers have devised low mechanical index (MI) pulse sequence schemes which enhance the detection of microbubble contrast even at MI’s below 0.2, and cancel out any tissue signals.
This is the recommended technique for imaging microbubbles, whether performed for the FDA approved indication of left ventricular opacification, or for perfusion imaging (Figure 2).
The improved basal segment attenuation with very low MI amplitude modulated pulse sequence schemes is because it can detect the non-linear fundamental frequency behavior of microbubbles at these very low MI imaging settings (<0.2), which are at <2.0 MHz frequencies, and thus have less far field attenuation.
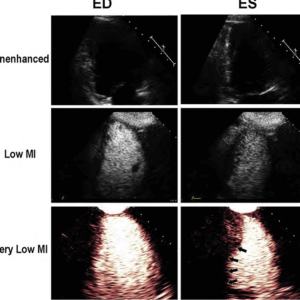
Figure 2. The unenhanced echo images in the upper panel at end diastole (ED) and end systolic (ES) are inadequate for regional wall motion analysis. The low MI harmonic images following the UEA injection are optimal around 0.3 MI, but still destroy apical cavity microbubbles and have basal segment attenuation (middle panels). When switching to amplitude modulated very low MI imaging at a MI <0.2, there is enhanced contrast from the same microbubbles and excellent basal segment visualization.
Targeted CEUS
Targeted contrast-enhanced ultrasound works in a similar fashion, with a few modifications. Microbubbles targeted with ligands that bind to certain molecular markers that are expressed by the area of imaging interest are still injected systemically in a small bolus. Microbubbles theoretically travel through the circulatory system, eventually finding their respective targets and binding specifically. Ultrasound waves can then be directed toward the area of interest. If a sufficient number of microbubbles have bound in the area, their compressible gas cores oscillate in response to the high frequency sonic energy field, as described in the ultrasound article. The targeted microbubbles also reflect a unique echo that stands in stark contrast to the surrounding tissue, owing to the discrepancy in orders of magnitude between microbubble and tissue echogenicity. The ultrasound system converts the strong echogenicity into a contrast-enhanced image of the area of interest, revealing the location of the bound microbubbles.[6] Detection of bound microbubbles may then show that a particular molecule is present in the area of interest, which can indicate a certain disease state, or identify particular cells in the area of interest.
Clinical Applications
Untargeted contrast-enhanced ultrasound is currently applied in echocardiography.
Improved Endocardial Resolution: Microbubbles can enhance contrast at the interface between tissue and blood. A clearer picture of this interface gives the clinician a better sense of the structure of an organ. Providing a clear image of issue structure is one of the most important functions of echocardiograms, as a thinning, thickening, or irregularity in the heart wall indicates a serious heart condition that requires either monitoring or treatment. The current 2018 Guidelines recommend using very low MI amplitude modulation sequences for this.
Blood Volume and Perfusion: Contrast-enhanced ultrasound allows technicians and physicians to (1) evaluate the degree of blood perfusion in an organ or area of interest and (2) evaluate the blood volume in an organ or area of interest.. Additionally, the relative intensity of microbubble echoes can also provide a quantitative estimate of blood volume.
Advantages
On top of the strengths of echocardiography, contrast-enhanced ultrasound adds these additional advantages:
- The body is 73% water and, therefore, acoustically homogeneous. Blood and surrounding tissues have similar echogenicities, so it is difficult to clearly discern the degree of blood flow and perfusion, or the interface between tissue and blood, using traditional ultrasound.
- Ultrasound imaging allows real-time evaluations of blood flow.
- Ultrasonic molecular imaging is safer than molecular imaging modalities, such as radionuclide imaging, because it does not involve radiation.
- Alternative molecular imaging modalities, such as MRI, PET, and SPECT are very costly. Ultrasound, on the other hand, is very cost-efficient and widely available.
- Since microbubbles can generate such strong signals, a lower intravenous dosage is needed. Micrograms of microbubbles are needed to perform contrast-enhanced ultrasounds compared to milligrams for other molecular imaging modalities, such as MRI contrast agents.
- Targeting strategies for microbubbles are versatile and modular. Targeting a new area only entails conjugating a new ligand.
Disadvantages
Contrast-enhanced ultrasound suffers from the following disadvantages:
- Microbubbles don’t last very long in circulation. They have low circulation residence times because they either get taken up by immune system cells or get taken up by the liver or spleen, even when they are coated with PEG.
- Microbubbles burst at low ultrasound powers (or mechanical indices), which is the measure of the acoustic power output of the ultrasound imaging system. Increasing MI increases image quality, but there are tradeoffs with microbubble destruction. There do not appear to be any significant harmful effects observed with the high MI impulses used to clear microbubbles from the microcirculation in humans.
Sign up for Updates
Lorem ipsum dolor sit amet, consectetur adipiscing elit ut aliquam, purus sit amet luctus venenatis magna.